A vapour is the gaseous state of a substance which is liquid or solid under ambient conditions. Vapours are often the “unseen enemy” when using the resin infusion process in composites. They are the unseen enemy because they aren’t visible in the mould release, in the fabric or the resin system at atmospheric pressure and only show their presence when either vacuum, or heat, or both are applied.
Vapours can show their presence by:
- Slowing down evacuation and giving the appearance of “false leaks”, causing time wastage attempting to fix leaks which don’t exist
- Causing porosity and, sometimes, seriously weakened parts.
Water vapour is the most common vapour encountered in composites and is always present to some extent. Other vapours may arise from resin system components, such as styrene and methyl ethyl ketone peroxide (MEKP).
Water vapour
Water from atmospheric humidity is either adsorbed onto the surface of impermeable materials or absorbed into the molecular structure of porous materials. This absorbed water is unseen and of no consequence at ambient temperature and pressure, but it can cause problems for the composite worker wanting to apply relatively high levels of vacuum to a composite layup.
The cause of the problem – water boils at ambient temperature when subject to vacuum
Absorbed water causes problems because it “boils” or converts into water vapour under reduced temperatures when vacuum is applied. The greater the vacuum, the lower the temperature at which the water boils. We learned at school that water boils at 100 0C or 212 0F. What our early teachers probably didn’t tell us is that this only occurs at standard atmospheric pressure of 1013 mbar or 29.92 inches of mercury (inHg). They probably didn’t tell us that the hottest cup of tea you can brew on the top of Mount Everest has a temperature of 72 0C (162 0F), which corresponds to an absolute pressure of 337 mbar or a vacuum of 20 inHg. And our early teachers certainly didn’t tell us that a typical composites industry rotary vane vacuum pump capable of achieving 10 mbar will boil water at around 8 0C or 46 0F.
We learned at school that water boils at 100 0C or 212 0F. What our early teachers probably didn’t tell us is that this only occurs at standard atmospheric pressure of 1013 mbar or 29.92 inches of mercury (inHg). They probably didn’t tell us that the hottest cup of tea you can brew on the top of Mount Everest has a temperature of 72 0C (162 0F), which corresponds to an absolute pressure of 337 mbar or a vacuum of 20 inHg. And our early teachers certainly didn’t tell us that a typical composites industry rotary vane vacuum pump capable of achieving 10 mbar will boil water at around 8 0C or 46 0F.
The relationship between vacuum (absolute pressure) and the boiling temperature of water is shown on the vapour pressure curve for water – see below.
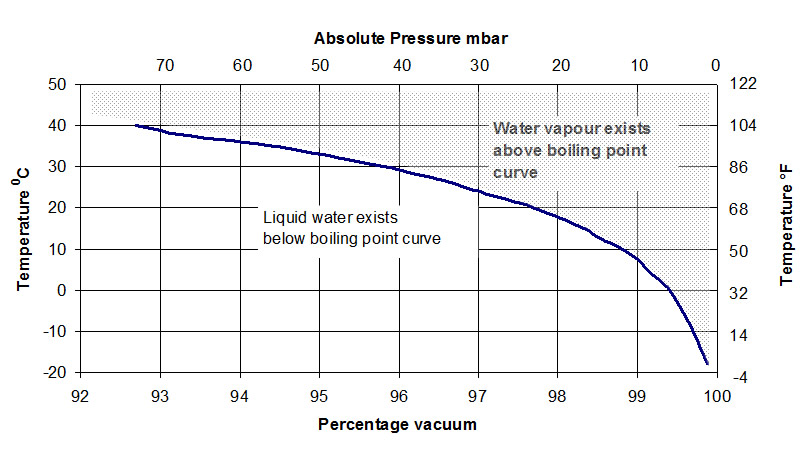
If you would like to observe this low temperature boiling phenomenon for yourself (and you have a vacuum pump capable of achieving about 10 mbar of absolute pressure), place a small amount of ambient temperature water in a resin trap with a viewport and apply vacuum to the trap. You will see that boiling will commence in accordance with the absolute pressure v. temperature curve. Do not be surprised if the water ceases to boil after some time. This will occur because boiling removes energy from the water (the latent heat of evaporation). This will lower the temperature of the remaining water, often to a temperature that would require an absolute pressure lower than the pump is capable of. You can verify the temperature reduction effect by feeling the temperature of the water when it is removed from the trap. If it has been boiling under vacuum, you will find that it has noticeably chilled.
Volume effect
OK, so a bit of absorbed water is boiled away under vacuum – isn’t that a good thing? Well, yes and no. It is probably a good thing from a resin adhesion perspective that a high enough vacuum boils the water away. The flip side of the coin is that a small amount of absorbed liquid water becomes a large amount of water vapour. If we extract figures from a chart called a “Steam Table” we find that either 1 kg or 1 lb of liquid water converts to the water vapour volumes shown in the table below:
| Absolute pressure (mbar) | Volume (of 1kg of water vapour) (m³) | Volume (of 1 lb of water vapour) (ft³) |
|---|---|---|
| 10 | 129 | 2,067 |
| 15 | 88 | 1,410 |
| 20 | 67 | 1,073 |
| 30 | 46 | 737 |
| 40 | 35 | 561 |
One kg of liquid water has a volume of 1 litre = 0.001 m³. At 20 mbar, which would be reasonably attainable in a leak tight vacuum bag, that 0.001 m³ of liquid water would expand to 67 m³ of water vapour – an expansion ratio of 67,000:1! Because the pumping capacity of a vacuum pump reduces significantly as it approaches its maximum vacuum level, removing that 1 kg of water vapour would take several hours of pumping for the 20 m³/h pump fitted to our 20/2 machine.
What does this mean in practical terms?
When the absolute pressure in the laminate reaches the vapour pressure point shown in the vapour curve (at the temperature of the laminate), the pumping rate will suddenly slow down if there is any water in the laminate. Without further checking, this apparent cessation of pumping may appear to be caused by a vacuum leak, or a vacuum pump problem. Therefore, when checking for vacuum leaks, always start the leak back test (or drop test) above the vapour pressure for the ambient temperature of the day. For example, if the ambient temperature is 20 0C (68 0F) begin the leak back test at an absolute pressure above 24 mbar (say, > 30 mbar). If the ambient temperature is 40 0C (104 0F), begin the leak back test above 73 mbar (say, > 80 mbar). You don’t need to be too precise about the starting pressure for the leak back test, as long as you are clearly above the vapour pressure point.
If a leak back test above the vapour pressure point is OK, but subsequent pumping down appears to stall at the pressure predicted by the water vapour pressure chart, you can be sure that the laminate contains some water. If you have a target end vacuum (absolute pressure) below the absolute pressure predicted by the vapour pressure chart for your ambient temperature, you have no choice but to keep pumping until the water has been evaporated away. This may take several hours and it is not uncommon for a big/thick part to require pumping overnight or more to reach the vacuum target.
point is OK, but subsequent pumping down appears to stall at the pressure predicted by the water vapour pressure chart, you can be sure that the laminate contains some water. If you have a target end vacuum (absolute pressure) below the absolute pressure predicted by the vapour pressure chart for your ambient temperature, you have no choice but to keep pumping until the water has been evaporated away. This may take several hours and it is not uncommon for a big/thick part to require pumping overnight or more to reach the vacuum target.
Materials with naturally high moisture contents can be difficult to pump down. Medium density fibreboard (MDF), which is sometimes infused for the manufacture of composite tooling, typically has a moisture content of around 7% by weight. Moisture levels this high can lead to very large amounts of water vapour being evolved and very slow pump-down times.
A reasonably natural conclusion from the foregoing would be to avoid evacuating the vacuum bag to below the vapour pressure point. Just vacuum down to some point above the vapour pressure point and leave the sleeping dog lie, so to speak. That would be ok if you are happy with the laminate quality achieved at that level of vacuum and if the temperature doesn’t rise during the course of the infusion because of a resin exotherm. If the exotherm causes the internal temperature to rise above the vapour pressure point, water vapour will inevitably be formed and you risk delamination of the laminate. This is a real risk with laminate fabrics which have not been stored in dry conditions and with naturally high moisture content materials such as Medium Density Fibreboard (MDF).
Practical hints
- Store raw materials for laminates in the driest possible conditions, to reduce the likelihood of water vapour problems occurring.
- Keep a copy of the vapour pressure curve handy, especially in warm climates. Use this to avoid wasting time looking for vacuum leaks, which aren’t leaks at all, because they are moisture problems. Always check for leaks at an absolute pressure above the vapour pressure point for your ambient temperature.
- Give the evacuation process “plenty of time”, especially for big/thick parts. A good guide to “plenty of time” is to monitor the absolute pressure when pumping down. If the pressure stalls for some time at the vapour pressure point, then resumes falling after pumping for some time, you will have removed the bulk of the water.
- If a material is known to have a high moisture content, don’t elevate its temperature while under vacuum, as this will produce an excessive quantity of water vapour and saturate the vacuum pump with water (see note below). Ideally, reduce the moisture content of wet materials before subjecting them to vacuum. If this isn’t possible, let the vacuum pump dry out the material while the material remains at ambient temperature.
How does water vapour affect the vacuum pump?
The problem with water vapour for a vacuum pump is that water vapour evaporated from the part wants to revert to liquid water again on being compressed back up to atmospheric pressure on the exhaust side of the pump. Just as atmospheric air always contains some water in gaseous form (atmospheric humidity); the air on the exhaust side of the pump can also hold some water in gaseous form. However, any excess water vapour in the exhaust region of the pump must condense into liquid water and emulsify in the pump oil. Apart from decreasing the lubricating properties of the oil, liquid water circulating in the pump creates a vicious circle within the pump. On the inlet side of the pump, where the absolute pressure is low, any water present in the oil will convert into water vapour and tend to fill the inlet cavity of the pump. As the pressure rises towards the exhaust port, the water vapour compresses back into liquid water which is transferred back to the inlet, where it converts back to water vapour and so on and on…
When saturated with water, a vacuum pump completely loses its capacity to pump anything else – it simply pumps the same amount of water round and round.
The vacuum pumps we use in our Vacmobiles are specifically selected to have the highest practicable water vapour tolerance. This is achieved in 2 ways:
- The pumps are deliberately made to run at elevated temperatures – in the vicinity of 90 0C to 110 0C (194 0F to 230 0F).
- The pumps are fitted with an external air admit port, called a gas ballast. This port admits clean air from outside the pump into the exhaust cavity of the pump. Because this air will not be fully saturated with water at the pump’s elevated operating temperature, the gas ballast air will be able to absorb some water vapour and hold it in vapour form until safely discharged from the pump. The gas ballast air enters the pump through a small external filter – usually situated on top of the pump. For the gas ballast to function, this filter must be kept clean (or replaced if blocked).
Solvent vapours from infusion resins
Also of possible concern when using the resin infusion process are vapours released from volatile resin components. Potential candidates for solvent vapour problems, particularly in warm climates, are styrene and initiators such as MEKP. Because of the wide range of raw materials used for resin manufacture the vapour pressure of mixed resins will be specific to an individual resin/hardener recipe. We recommend that users of the infusion process ask their resin supplier to provide a vapour pressure versus temperature curve for the mixed resin system you intend to use. If your supplier is unable to provide this information, conduct the following test in a resin trap – ideally one with a viewport and an absolute pressure gauge attached. Alternatively use a transparent vessel that will safely withstand vacuum (but not a glass jar, unless safely shielded).
- Mix up a small batch of the resin you propose to use at the highest ambient temperature you are likely to encounter when using that particular resin and place it in the resin trap
- Increase the vacuum slowly
- If your trap has a viewport, observe the resin mixture. Initially, you will see entrained air bubbling out of the resin. However, once the entrained air has been completely removed, the bubbling should cease. If the bubbling does not cease and even becomes more vigorous with increasing vacuum, one or more solvent components have commenced boiling.
- If able to observe the process, reduce the vacuum level until the resin ceases boiling and note the absolute pressure at which this occurs. This pressure will be the lowest absolute pressure you can apply whenever that particular resin system is present in liquid form.
If unable to observe the process because you don’t have a resin trap with a viewport, conduct the first 3 steps as above and leave the resin under vacuum until it has cured. Examine the cured resin for porosity and brittleness. If badly porous and easily broken, the vacuum level has been too high. Repeat the experiment, but stop at a poorer vacuum and again observe the cured resin. Continue reducing the vacuum level until the cured resin shows no signs of porosity.
Summing up
- Liquids can vaporise into a large volume of gas, even at ambient temperature, if a high enough vacuum is applied. The gas thus produced can slow evacuation, give the appearance of a “false” leak and ultimately cause porosity and weakness in the part.
- The troublesome liquids can be water from atmospheric humidity or solvents from resin systems
- If concerned about vapour problems in composites, it pays to understand the concept of absolute pressure and to have a way of measuring it. Refer to Vacman’s Note – “Absolute pressure – turning vacuum upside down”
- If vapours are present in any significant quantity, there is a likelihood they will contaminate the oil in the vacuum pump. Inspect the oil in the vacuum pump’s sight glass regularly. If no longer transparent and gold in colour, change it. Refer to Vacman’s Note – “Oil changing and dust removal – the essentials of vacuum pump care.”
Feedback or queries on this note?
We are keen to improve the accuracy and value of Vacman’s Notes. If you have any feedback or queries regarding this note, or would like to suggest new topics to be covered, Vacman would be pleased to hear from you! Please comment below! Or email [email protected]

